

Consult Instructions For Use
Use by
Sterilization: Ethylene-Oxide Gas
Do not reuse
Package Contents
Reference Number
Lot Number
CAUTION: U. S. Federal law restricts this device to sale by or on the order of a physician
Manufacturer
Do not resterilize
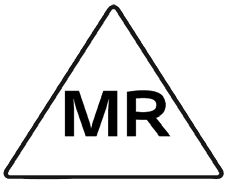
MRI conditional
Borvo Medical, Inc.
2500 Old Middlefield Way, Ste E
Mountain View, CA 94043
Device Description
The Borvo EVACTM is indicated to facilitate the evacuation of a chronic or subacute hematoma or hygroma in the cranium. The EVAC is intended for draining of fluid accumulated in the subdural space, including chronic or subacute hematomas and hygromas. The EVAC is also intended for removing air and fluid from the subdural space following open surgical procedures to remove subdural collections.
EVAC is available as a standalone kit or shipped with an accompanying cranial access kit.
Refer to the product package label for a list of included components.
Each EVAC kit contains:
Drainage port
Surgical tubing
Negative pressure reservoir
Indications
The EVAC is indicated to facilitate the evacuation of a chronic or subacute hematoma or hygroma in the cranium. The EVAC is intended for draining of fluid accumulated in the subdural space, including chronic or subacute hematomas and hygromas. The EVAC is also intended for removing air and fluid from the subdural space following open surgical procedures to remove subdural collections.
Contraindications
EVAC is contraindicated for treatment of acute hematomas and use in patients undergoing anticoagulant therapy. EVAC is not sold or intended for use except as indicated herein.
Warnings and Precautions
EVAC is a single use product. It should not be reused, reprocessed, or resterilized, which could result in contamination of the device resulting in injury, illness, or death of the patient. Borvo Medical is not responsible for any product that has been reused, reprocessed, or resterilized.
If any EVAC component is expired at the time of use, it should be discarded and replaced with an in-date equivalent.
The EVAC device is made of titanium and should not be used on any patient with a titanium allergy.
EVAC and its components are latex free.
Appropriate aseptic technique must be maintained during the EVAC procedure and during subsequent insertion site maintenance/dressing changes.
Placement of the EVAC port is a surgical procedure that risks penetrating the cerebrum. It must be performed by qualified physicians with appropriate technique and skill.
EVAC is made to be used only with a 5.31mm drill bit. Failure to use the appropriately sized drill bit may cause injury.
The burr hole must be kept free of loose body contamination during introduction of the EVAC to ensure an adequate seal for function of the negative pressure reservoir and system performance.
Sterility is required during all aspects of the EVAC placement.
Care should be taken to prevent contamination of the burr hole, EVAC, negative pressure reservoir and other components by loose bodies, particulates and residues from surgical gloves and garments, which can cause allergic reaction or injury.
If the patient exhibits adverse symptoms that may be caused by irritation, contamination, infection, or allergic reaction, the EVAC should be removed.
Patient supervision and monitoring during the drainage procedure is essential. Failure of timely diagnosis of neurological deficiency may result in injury.
Appropriate protection of the insertion site is essential to prevent pullout of the EVAC or disconnection of the tubing connecting the negative pressure reservoir.
Care should be taken to avoid kinking or obstruction of the tubing, which may impair the performance of the negative pressure reservoir.
Care should be taken to avoid over- or under-insertion of the port.
Blockage of the burr hole, tubing or EVAC may occur. When this occurs and additional fluid remains to be evacuated placement of an additional EVAC or craniotomy may be considered.
Cranial access kits shipped with EVAC devices contain separate Instructions for Use documentation and should be operated per those instructions.
Instructions for Use
The steps described below are a general guideline. Treating physicians may adapt or change the procedure based on their personal training, clinical experience, and judgment. Prior to use, it is necessary for all responsible personnel to understand the use and function of the EVAC. Maintenance of sterility during use of this product is essential. Sterile technique should be employed at all times.
Selection and Access of the Drainage Site, Introduction of the EVAC, and Performance of Drainage
1. Local or general anesthesia may be utilized.
2. Image the suspected area with computed tomography (CT scan) or MRI (Magnetic Resonance Imaging) to identify the area of greatest subdural fluid accumulation to use as the insertion site.
3. Follow the Instructions for use of the Cranial Access Kit to be used for the procedure. Make sure to use a 5.31mm drill bit for the burr hole.
4. After incising the dura, remove all exposed dura and subdural membrane tissue from the burr hole. Utilize a scalpel and forceps or a unipolar cautery. If cauterizing, bend the tip of the unipolar cautery ~90° and cauterize around the undersurface of the burr hole.
WARNING: It is necessary to clear the dura and underlying membranes from the burr hole and observe fluid flowing freely from the burr hole before inserting the EVAC.
5. Remove the self-retaining scalp retractor, if in place, and insert the evacuating port by screwing in a clockwise direction. Up to four (4) complete turns is sufficient to create a firm placement and seal in the skull.
WARNING: Inserting the EVAC beyond the inner table of the skull may cause injury.
6. Attach one end of the tubing to the EVAC and the other end to the negative pressure reservoir.
7. Squeeze the negative pressure reservoir and cap the one-way valve to create a consistent suction.
8. Close the wound around the EVAC. Utilize antiseptic ointment around the insertion point of the EVAC and dress with sterile dressing.
9. Complete removal of fluid is typically achieved within 24 hours. The negative pressure reservoir should be monitored. If it fills, empty and reapply suction as needed, using typical sterile technique.
WARNING: During evacuation a blood clot, subdural membrane, or loose bone or tissue fragments may clog the EVAC, tubing, or negative pressure bulb. When this occurs and additional fluid remains to be evacuated placement of an additional EVAC or craniotomy may be considered.
10. Multiple EVACs may be utilized simultaneously at the physician’s discretion.
WARNING: The use of multiple evacuating ports cumulatively may exceed the USP Bacterial Endotoxin exposure limit.
11. When the procedure is completed, dispose of kit components appropriately.
12. MRI Safety Information:
MRI Safety Information
The Endoport Vacuum-Assisted Collection (EVAC) is MR Conditional. A patient with the EVAC may be safely scanned under the following conditions. Failure to follow these conditions may result in injury to the patient.
MR Conditional
Parameter
Conditions of Use/Information
Nominal Values of Static Magnetic Field (T)
1.5-Tesla or 3.0-Tesla
Maximum Spatial Field Gradient (T/m and gauss/cm)
40-T/m (4,000-gauss/cm)
Type of RF Excitation
Circularly Polarized (CP) (i.e., Quadrature- Transmission)
Transmit RF Coil Information
There are no transmit RF coil restrictions. Accordingly, the following may be used: body transmit RF coil and all other RF coil combinations (i.e., body RF coil combined with any receive-only RF coil, transmit/receive head RF coil, transmit/receive knee RF coil, etc.)
Operating Mode of MR System
Normal Operating Mode
Maximum Whole Body Averaged SAR
2-W/kg (Normal Operating Mode)
Limits on Scan Duration
Whole body averaged SAR of 2-W/kg for 60 minutes of continuous RF exposure (i.e., per pulse sequence or back-to-back sequences/series without breaks)
MR Image Artifact
The presence of this implant produces an imaging artifact. Therefore, carefully select pulse sequence parameters if the implant is located in the area of interest.
Procedure for removing EVAC
Use of EVAC Following Craniotomy Drainage Procedure
Air and fluid may be evacuated with the EVAC after a craniotomy procedure performed to remove a chronic or subacute subdural hematoma. Before using the EVAC as a post craniotomy drain, review the instructions above for Selection and Access of the Drainage Site, Introduction of the EVAC, and Performance of Drainage.
1. Select an appropriate site for insertion within close proximity to the bone flap. To lower the risk of bone flap contamination, do not attempt to drill a hole or insert the evacuating port directly into the bone flap.
2. Follow the Instructions for use of the Cranial Access Kit to be used for the procedure. Make sure to use a 5.31mm drill bit for the burr hole.
3. After incising the dura, remove all exposed dura and subdural membrane tissue from the burr hole. Utilize a scalpel and forceps or a unipolar cautery. If cauterizing, bend the tip of the unipolar cautery ~90° and cauterize around the undersurface of the burr hole.
WARNING: It is necessary to clear the dura and underlying membranes from the burr hole and observe fluid flowing freely from the burr hole before inserting the EVAC.
4. If using a self-retaining scalp retractor, remove it at this point and insert the EVAC by screwing in a clockwise direction. Up to four (4) complete turns is typically sufficient to create a firm placement and seal in the skull.
WARNING: Inserting the EVAC beyond the inner table of the skull may cause injury.
5. Attach one end of the tubing to the EVAC and the other end to the negative pressure reservoir.
6. Squeeze the negative pressure reservoir and cap the one-way valve to create a consistent suction.
7. Close the wound around the EVAC. Utilize antiseptic ointment around the insertion point of the EVAC and a dress with sterile dressing.
8. Complete removal of fluid is typically achieved within 24 hours. The negative pressure reservoir should be monitored. If it fills, empty and reapply suction as needed to continue fluid evacuation.
WARNING: During evacuation a blood clot, subdural membrane, or loose bone or tissue fragments may clog the EVAC, tubing, or negative pressure bulb. When this occurs and additional fluid remains to be evacuated placement of an additional EVAC or craniotomy may be considered.
9. Multiple EVACs may be utilized simultaneously at the physician’s discretion.
WARNING: The use of multiple evacuating ports cumulatively may exceed the USP Bacterial Endotoxin exposure limit.
10. When the procedure is completed, dispose of kit components appropriately.
How Supplied
The EVAC and tubing are supplied sterile inside a sealed pouch. The negative pressure reservoir is supplied sterile separately packaged. If a cranial access kit is included, it is supplied sterile in a separate box. Refer to applicable labeling on the outside of all packaging. All supplied products are intended for Single Use Only. No components should be re-sterilized or reused. After use, all components should be disposed of appropriately.
All component products are sterilized with ethylene oxide gas. All sealed packaging should be inspected for integrity. Do not use if any component package that appears opened or damaged.
Patient Education
The treating physician is responsible for informing patients and/or their legal representative(s) about the EVAC procedure, including relevant risks, warnings, precautions, and alternative treatments.
Complications
Risks of the EVAC procedure include bleeding, damage to brain tissue, seizures, stroke, or death. Additionally complications that may also occur include adverse reaction to foreign bodies; bacterial contamination, wound abscess, fistula formation, and herniation of tissue of the surgical site; pneumocephalus; inadequate evacuation due to improper incising of the dura or placement of the EVAC; obstruction of the system by kinking the tubing or plugging the tubing or port with blood clots or bone particles; and, disconnection of the system.
Additional surgical procedures such as placement of an additional EVAC and/ or craniotomy may be required due to persistent or recurring subdural effusion.
Acceptance and Returns
Products shall be deemed to be accepted upon delivery to the customer (“Customer”) unless such products are shipped in error, and Borvo shall not accept the return of any product unless the product is shipped in error or is under warranty. Products shipped in error will be replaced with the correct product, and products returned under warranty will be replaced at Borvo’s discretion.
All orders are nonrefundable. In the case where the incorrect product was shipped in error or the product is under warranty, the customer must contact Borvo Customer Service in writing within twenty (20) business days after delivery to receive a return material authorization (“RMA”) number and to arrange return shipment to Borvo at Borvo’s expense. The RMA must be clearly written on each box or return label. Products shipped in error must be returned prior to use in the original packaging.
Warranties
Borvo shall have no liability for products returned by Customer as to which Borvo’s examination discovers that: i) the product has been exposed to unusual or excessive environmental, mechanical, electrical or thermal stress; or ii) the product was not maintained in the manner described in any applicable instructions for use or specifications, or iii) product malfunction is the result of misuse, abuse, improper application, alteration, accident, or negligence in use, storage, transportation, handling, or if the original identification markings on the product have been removed, defaced or altered; or iv) the product was purchased from an unauthorized source, or v) the product is classified as other than a commercial production unit, e.g., a design verification unit, sample, preproduction unit, developmental unit, or prototype unit; or (vi) the defect or deficiency was caused by external factors beyond Borvo’s reasonable control. All warranty claims are subject to verification by Borvo. The foregoing warranty extends to the Customer only. In the event that the returned Product is not defective or does not otherwise qualify for warranty coverage, the Customer must reimburse Borvo for all shipment costs. Moreover, in the event that Borvo issues an RMA and ships a replacement product to the Customer prior to receiving the returned Product, the Customer must return the allegedly defective Product to Borvo within forty-five (45) days of receiving the replacement Product or else the full price of the replacement Product will be invoiced to the Customer. Further, if Borvo ships a replacement product to the Customer prior to receiving the returned Product and subsequently determines that the returned Product is not defective or does not otherwise qualify for warranty coverage, the Customer must pay the full price for the replacement Product that Borvo shipped to the Customer. The non-defective Product will be returned to the Customer at the Customer’s expense.
NO OTHER EXPRESS OR IMPLIED WARRANTIES, INCLUDING IMPLIED WARRANTIES OF NON‐INFRINGEMENT, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, QUIET ENJOYMENT, SYSTEM INTEGRATION AND DATA ACCURACY, WILL APPLY.
Limitation of Liability
NOTWITHSTANDING THE FOREGOING, BORVO’S LIABILITY ARISING OUT OF OR RELATED TO (I) ANY GOODS PURCHASED UNDER THIS AGREEMENT; AND (II) SERVICES PROVIDED UNDER THIS AGREEMENT AND/OR SALE SHALL BE LIMITED TO REFUND OF THE PURCHASE PRICE OF SUCH GOODS OR SERVICES. IN NO EVENT SHALL BORVO BE LIABLE FOR LOST USE, PROFITS, GOODWILL, REVENUE, COST OF PROCUREMENT OF SUBSTITUTE GOODS, OR ANY OTHER SPECIAL, INDIRECT, RELIANCE, INCIDENTAL OR CONSEQUENTIAL DAMAGES HOWEVER CAUSED AND UNDER ANY THEORY OF LIABILITY. THE FOREGOING LIMITATIONS SHALL APPLY REGARDLESS OF WHETHER BORVO HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES, NOTWITHSTANDING THE FAILURE OF ESSENTIAL PURPOSE OF ANY LIMITED REMEDY AND REGARDLESS OF WHETHER SUCH DAMAGES ARISE OUT OF THIRD-PARTY CLAIMS AGAINST CUSTOMER.
